BSC 5th Sem ( P-2 ) BASIC ATOMIC MODELS
Atom Model
A theoretic explanation for the structure of atom is called an atom model.To account for the experimentally observed spectroscopic data at that time, several theories have been proposed from time to time regarding the atomic structure which are known as atomic models. The various models are:
1. Thomson's plum pudding model
2. Rutherford's nuclear model
3. Bohr's model
4. Summer fields relativistic model
5. Vector model
6. Wave mechanical model
Thomson's Plums Pudding Model
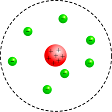
J.J. Thomson gave the first explanation about the arrangement of positive charges and the electrons inside the atom. According to him at atom is a sphere of positive charges having radius of order of 10-10 m. The positive charge is uniformly distributed over the entire sphere and the electrons are embedded in the sphere of positive charges just like seeds in a watermelon or plums in the pudding. For this reason, Thomson's atom model is known as plum-pudding model. The total positive charge inside the atom is equal to the total negative charge so it is electrically neutral.
• It could not explain the origin of the spectral lines.
• It could not account for the scattering of a-particles.
Rutherford's Atom Model
Ruther for suggest the following picture of the atom : (using a-particle scattering).
1. An atom may be regarded as a sphere of
diameter in a small role of the positive charge and almost the entire mass of
the atom is concentrated in a small central core called nucleate (diameter of
about 10 m) Fig.
2. The nucleus is surrounded by electrons .
3. As atom is electrically neutral, the total positive charge of the nucleus is equal to the total negative charge of the electrons in the atom.
Drawbacks:
( i) The stability of the atom as a whole.
(ii) Distribution of electrons outside the nucleus.
Theory of alpha - Particle Scattering
We shall assume the following:
1. The a-particle and the nucleus are point charges.
2. The scattering is due to coulomb electrostatic repulsive forces between th o-particle and the positive charge of the nucleus (Ze).
3. The nucleus is so massive compared with the a-particle that it does not move during their
interaction.
4. The a-particles do not penetrate to nuclear region and the strong interaction nuclear forces are not involved.
5. Let r be the instantaneous separation between a-particle and nucleus. Owing to he variation of the electric force with 1/r, the a-particle's path is a hyperbola with the nucleus at the outer focus (Fig. 1.3)