BSC 5th SEM QUANTUM MECHANICS - I NOTES
Quantum Mechanics
The Failure of Classical Physics
Even though most phenomena that we can in everyday life are explained using classical mechanics, there are some cases where classical mechanics fails to give a proper/correct explanation. Some of the examples showcasing the failure of classical mechanics are:
1. Hydrogen atom (Stability of an atom):
Electron revolving around the hydrogen nucleus are constantly changing direction and hence accelerating. According to classical mechanics, an accelerating charged particle should emit electromagnetic radiation and hence lose energy. Hence, the electrons would eventually lose all energy and crash into the nucleus. But, we know that this is not the case.
2. Atomic Spectra:
A classical model would have electrons orbiting the nucleus like little planets. They could, classically, be at any radius. They could also, classically, lose or be given any amount of energy to change the orbit.
3. Blackbody radiation:
According to classical mechanics, electromagnetic rays radiated from the black body can have any wavelength. This is actually not true as the range of wavelength is dependent on the frequency (wavelength) of the electromagnetic wave and they are emitted as discrete packets of radiation known as photons.
4. Photoelectric effect:
In this phenomenon, light incident on the metal causes electron emission. According to the classical mechanics, the energy in electromagnetic waves is continuous and depends on the intensity of light, hence, a dim light has discrete energies dependent on its frequency.
5. Compton Effect:
The Compton effect is the term used for an unusual result observed when X-rays are scattered on some materials. By classical theory, when an electromagnetic wave is scattered off atoms, the wavelength of the scattered radiation is expected to be the same as the wavelength of the incident radiation. This cannot be explained with the classical theory.
6. Specific heat of solids:
According to classical mechanics specific heat of solids is constant and independent of temperature. But experimental results show that this depends on temperature, this can be explained by quantum statistics.
7. Planck’s quantum theory:
According to Planck’s quantum theory,
1. Different atoms and molecules can emit or absorb energy in discrete quantities only. The smallest amount of energy that can be emitted or absorbed in the form of electromagnetic radiation is known as quantum.
2. The energy of the radiation absorbed or emitted is directly proportional to the frequency of the radiation.
Quantum mechanical explanation for failures of classical physics:
Quantum mechanical explanation for failures of classical physics:
Photoelectric effect- Einstein’s explanation:
Einstein said, is a beam of particles whose energies are related to their frequencies according to Planck's formula. When that beam is directed at a metal, the photons collide with the atoms. If a photon's frequency is sufficient to knock off an electron, the collision produces the photoelectric effect.
Einstein’s Explanation of the Photoelectric Effect
The strength of the photoelectric current depends upon the intensity of incident
radiation, and it should be higher than the threshold frequency.
The reverse stopping potential was the photo-current stop. It is independent of the
intensity of incident radiation.
Photoelectric current does not occur if the frequency of the incident radiation is below
the threshold frequency. A metallic strip, when exposed to light or sun, will not be
able to produce the Photoelectric effect unless the frequency is greater than the
threshold value.
The photoelectric effect is an instantaneous process. As soon as light hits the surface,
the electrons of the metal come out.
Einstein’s Equation of the Photoelectric Effect
According to the Einstein-Planck relation, Einstein explained the photoelectric effect
based on Planck’s quantum theory, according to which, light radiation travels in the form of
discrete photons. The energy photon is hv , where h is constant and v is the frequency of light
.
E = hν
Where 'h' is Planck's constant = 6.6261 × 10-34 Js. and 'ν' is the frequency of the emitted radiation.
Compton Effect:
Monochromatic X-rays with wavelength λ are incident on a sample of graphite (the
“target”), where they interact with atoms inside the sample; they later emerge as scattered X-rays with wavelength λ′. A detector placed behind the target can measure the intensity of
radiation scattered in any direction θ with respect to the direction of the incident X-ray beam.
This scattering angle θ, is the angle between the direction of the scattered beam and the
direction of the incident beam. In this experiment, we know the intensity and the wavelength λ of the incoming (incident) beam; and for a given scattering angle θ, we measure
the intensity and the wavelength λ′ of the outgoing (scattered) beam. Typical results of these
measurements are shown in Figure, where the x-axis is the wavelength of the scattered X-ray's and the y-axis is the intensity of the scattered X-rays, measured for different scattering
angles (indicated on the graphs). For all scattering angles (except for θ=0°), we measure two
intensity peaks. One peak is located at the wavelength λ, which is the wavelength of the
incident beam. The other peak is located at some other wavelength, λ′. The two peaks are
separated by Δλ, which depends on the scattering angle θ of the outgoing beam (in the
direction of observation). The separation Δλ is called the Compton shift.
The relation for the Compton shift:
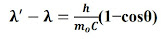
Ө: the angle at which radiation scattered.
m0 : rest mass of an electron.
h/m0c : Compton wavelength of the electron.
Stability of atom and atomic spectra:
Stability of atom:
Atoms in quantum mechanics don’t suffer from the same radiation problem as atoms in classical mechanics. A quantum system exists in many instances that can interfere with one another on a small scale. As a result, on an atomic scale an electron doesn’t have a trajectory and so it can’t be said to accelerate and it doesn’t radiate. In addition, when the probability of finding an electron is highly peaked at a particular location, quantum mechanics makes the instances spread out. The potential produced by the nucleus pulls the electron instances toward the nucleus. Atoms can be stable because the spreading out produced by quantum mechanics and the attraction produced by the potential balance out.
Atomic spectra:
Because the arrangement of energy levels is unique for each type of atom, the characteristic set of frequencies of light emitted by an atom when it undergoes transitions is also unique and so can be used to identify the atom. These characteristic sets of frequencies for different atoms are known as atomic spectra.
de Broglie’s hypothesis of matter waves:
Whenever a particle is in motion, waves are associated with it. These wave are called matter waves or de-Broglie waves.
a) de Broglie’s wavelength interms of momentum:
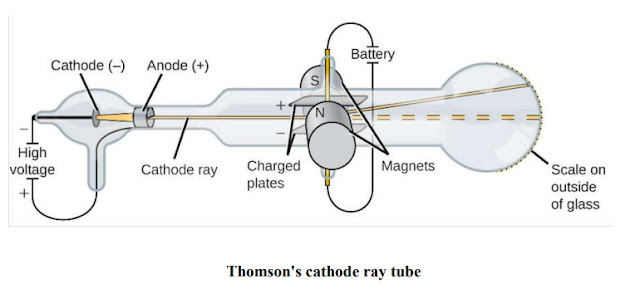
Thomson Experiment: Discovery of the electron and nucleus
J.J. Thomson began experimenting with cathode
ray tubes. Cathode ray tubes are sealed glass, tubes A high voltage is applied across two electrodes at one end of the tube, a beam of particles to flow from the cathode to the
anode The tubes are called cathode ray tubes.
The ray can be detected by painting
a material known as phosphors ,
To test the properties of the particles, Thomson placed two oppositely-charged electric plates
around the cathode ray. The cathode ray was deflected away from the negatively-charged
electric plate and towards the positively-charged plate. This indicated that the cathode ray
was composed of negatively-charged particles.
Thomson also placed two magnets on either side of the tube, and observed that this magnetic
field also deflected the cathode ray.
Thomson repeated his experiments using different metals as electrode materials, and
found that the properties of the cathode ray remained constant no matter what cathode
material they.
. From this evidence, Thomson made the following conclusions:
the velocity of the beam. Thus,

Where,
m & e = mass and charge of the electron
d = distance between N & S
E & B = Electric & Magnetic field
Each of the quantities in the above expression was measured so the
The plum pudding model:
Thomson knew that atoms had an overall neutral charge. Therefore, he reasoned that there must be a source of positive charge within the atom to counterbalance the negative charge on the electrons. This led Thomson to p negative particles floating within a soup of diffuse positive charge. This model is often called the plum pudding model of the atom, due to the fact that its description plum pudding.
Wave packets:
Superposition of two or more waves with slightly different wavelength interfere constructively where the particle is present and destructively where the particle is absent is called Wave packets. (Waves can be in a group and such groups are called wave packets).
Phase velocity :
The velocity with which the phase of a wave travels is called phase velocity. (velocity of the single wave).
Group velocity :
The velocity with which a wave packet travels is called group velocity. (velocity of the many wave)
YOU CAN MORE NOTES AND DOWNLOAD BELOW LINK